Jennifer L. Sherr , Lori M. Laffel , Jingwen Liu , Wendy Wolf , Jeoffrey Bispham , Katherine S. Chapman , Daniel Finan , Lina Titievsky , Tina Liu , Kaitlin Hagan , Jason Gaglia , Keval Chandarana , Richard Bergenstal , Jeremy Pettus; Severe Hypoglycemia and Impaired Awareness of Hypoglycemia Persist in People With Type 1 Diabetes Despite Use of Diabetes Technology: Results From a Cross-sectional Survey. Diabetes Care 20 May 2024; 47 (6): 941–947. https://doi.org/10.2337/dc23-1765
Download citation file:
toolbar searchTo determine how diabetes technologies, including continuous glucose monitoring (CGM) and automated insulin delivery (AID) systems, impact glycemic metrics, prevalence of severe hypoglycemic events (SHEs), and impaired awareness of hypoglycemia (IAH) in people with type 1 diabetes in a real-world setting within the U.S.
RESEARCH DESIGN AND METHODSIn this retrospective, observational study with cross-sectional elements, participants aged ≥18 years were enrolled from the T1D Exchange Registry/online community. Participants completed a one-time online survey describing glycemic metrics, SHEs, and IAH. The primary objective was to determine the proportions of participants who reported achieving glycemic targets (assessed according to self-reported hemoglobin A1c) and had SHEs and/or IAH. We performed additional subgroup analyses focusing on the impact of CGM and insulin delivery modality.
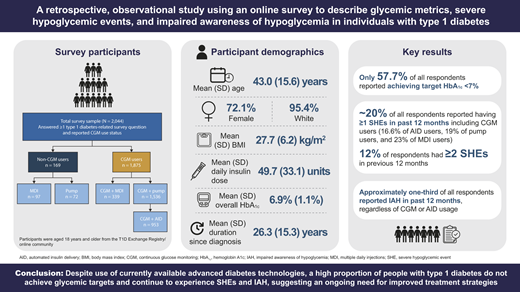
A total of 2,074 individuals with type 1 diabetes were enrolled (mean ± SD age 43.0 ± 15.6 years and duration of type 1 diabetes 26.3 ± 15.3 years). The majority of participants (91.7%) were using CGM, with one-half (50.8%) incorporating AID. Despite high use of diabetes technologies, only 57.7% reported achieving glycemic targets (hemoglobin A1c <7%). SHEs and IAH still occurred, with ∼20% of respondents experiencing at least one SHE within the prior 12 months and 30.7% (95% CI 28.7, 32.7) reporting IAH, regardless of CGM or AID use.
CONCLUSIONSDespite use of advanced diabetes technologies, a high proportion of people with type 1 diabetes do not achieve glycemic targets and continue to experience SHEs and IAH, suggesting an ongoing need for improved treatment strategies.


Type 1 diabetes affects more than 8.7 million people globally (1). For more than 100 years exogenous insulin therapy has been considered the standard of care for people with type 1 diabetes. The objective of type 1 diabetes treatment has been to maintain blood glucose concentrations within a specified range to achieve glycemic targets with glucose levels as close as possible to the reference range in those without diabetes (2–5). Despite the best efforts of individuals with diabetes and their care teams, many people with type 1 diabetes remain unable to reach glycemic targets, with an estimated one in four meeting the American Diabetes Association/European Association for the Study of Diabetes consensus target goal of hemoglobin A1c (HbA1c) 1c targets should be set for those in whom they can be safely used, a recent global study of nearly 4,000 individuals with type 1 diabetes reported that a target of
Exogenous insulin has a narrow therapeutic window, and an excess of insulin relative to physiologic requirements can result in hypoglycemia (2,5,7). If untreated, severe hypoglycemic events (SHEs), events that require assistance from another individual to provide rescue from the event, can result. SHEs have been associated with loss of consciousness, collapse, seizure, injury, and even death (7,8). Recurring SHEs lead to a decrease in quality of life, as well as increased morbidity and mortality (8). Importantly, the occurrence of SHEs has also been linked to a higher risk for falls, hospitalization, cardiovascular events, depression, and death in people with type 1 diabetes (9–11). It has been estimated that 4–10% of all deaths in people with type 1 diabetes are attributable to SHEs (12,13).
Recurrent hypoglycemic events can lead to a reduction in counterregulatory hormone responses resulting in impaired awareness of hypoglycemia (IAH) (14). IAH may be reversed with strict avoidance of hypoglycemia (15). It has been estimated that IAH occurs in up to 40% of people with type 1 diabetes (8,16–19) and is associated with a three- to sixfold increase in the risk of SHEs (16,18).
Advances in diabetes management technologies, including continuous glucose monitoring (CGM) and automated insulin delivery (AID) systems (such as hybrid closed-loop systems), have improved clinical management of type 1 diabetes (20). Clinical trials have demonstrated that CGM and AID systems can increase time in target glucose range of 70–180 mg/dL and lead to lower HbA1c levels, while concomitantly reducing the frequency of hypoglycemia (20). As a result, CGM and other technologies are now recognized as standard of care for people living with type 1 diabetes. However, the impact of these diabetes technologies on the frequency of SHEs and IAH, and achievement of glycemic targets, in real-world settings is unclear.
In this study, we characterize the self-reported prevalence of SHEs and IAH in individuals with type 1 diabetes according to use of CGM and/or insulin pumps, including AID systems, while concurrently assessing glycemic metrics using self-reported HbA1c.
The primary study objectives were to estimate 1) glycemic metrics as well as the proportion of participants meeting glycemic targets (i.e., self-reported HbA1c <7%), 2) the proportion of participants reporting SHEs and the frequency of SHEs, and 3) the proportion of participants with IAH (as assessed with modified Gold score) across the use of different diabetes management approaches and technologies.
This was an observational, retrospective study with cross-sectional elements. An online survey was used for description of glycemic metrics, SHEs, and IAH in adults with type 1 diabetes, with stratification by glucose monitoring (CGM vs. non-CGM) and insulin delivery method (multiple daily injections [MDIs], conventional pump, or AID systems) (Fig. 1 and Supplementary Table 1). Use of AID was designated based on participant’s indication of using an insulin pump with a hybrid closed-loop mode. Participants were enrolled from the T1D Exchange Registry (21,22) and T1D Exchange online communities and were asked to complete a one-time survey via the online platform Qualtrics (Supplementary Material).

Study schema: observational, cross-sectional study with enrollment of adults (aged ≥18 years) with a diagnosis of type 1 diabetes for ≥2 years from the T1D Exchange Registry or online communities. *CGM + pump subgroup includes participants who used CGM and conventional pumps (n = 574), those with unknown pump type (n = 9), and those who used CGM and AID pumps (n = 953).

Study schema: observational, cross-sectional study with enrollment of adults (aged ≥18 years) with a diagnosis of type 1 diabetes for ≥2 years from the T1D Exchange Registry or online communities. *CGM + pump subgroup includes participants who used CGM and conventional pumps (n = 574), those with unknown pump type (n = 9), and those who used CGM and AID pumps (n = 953).
Eligible participants were aged ≥18 years and had a diagnosis of type 1 diabetes for ≥2 years. Participants were willing and able to provide informed consent, respond to survey questions in English, and were residents of the U.S. The subset of participants who contributed CGM data were currently using CGM, had data available for ≥3 months before enrollment, and had used CGM for ≥5 days per week in the 3 months prior to enrollment. Those not eligible included women who were currently pregnant or who had given birth in the previous 12 months and individuals who were enrolled in an interventional clinical study.
The study was conducted in accordance with the current Guidelines for Good Pharmacoepidemiology Practices and in accordance with local applicable laws and regulations and underwent institutional review board approval before implementation of any study procedures. Prior to providing Web-based consent, eligible participants received information regarding the purpose and potential benefits/risks of this study; participants then provided Web-based consent authorizing the use and disclosure of personal health information as described in the informed consent form.
Participants provided demographic data, baseline clinical characteristics, and information on CGM use and insulin delivery method through responses to questions in an online survey. The survey was created by a multidisciplinary team of experienced diabetes experts. The value and timing of participants’ most recent HbA1c measurements were self-reported and data on measurements were excluded from HbA1c summaries if obtained >12 months prior to the survey. Glycemic targets were self-reported HbA1c
The study aim was to enroll ∼2,000 participants with the expectation that 50% of participants would provide CGM data. Assuming a 10% rate of missing data, a sample size of 900 participants would produce a two-sided 95% CI with a half-width of no more than 3.3% for the primary outcomes of glycemic metrics, SHEs, and IAH. This level of precision was considered appropriate for descriptive statistics of primary outcome measures for CGM and non-CGM users.
The total survey sample, which was used for summarizing survey data, was defined as all individuals who completed at least one diabetes-related survey question and reported CGM use and insulin delivery method.
All analyses were descriptive and no statistical hypothesis testing was performed. Continuous variables were summarized with the number of participants, mean, SD, median, interquartile range, and minimum and maximum values. Categorical variables were summarized with the number and proportion of participants. Summary statistics and 95% CIs are reported. Comparisons were performed post hoc based on status of overlapping 95% CIs.
Summary data are reported for the overall participant sample and for cohorts based on CGM use and insulin delivery method: non-CGM users (both MDI and pump users; cohorts combined in analyses due to relative size of cohorts) and CGM users utilizing MDI (CGM + MDI), conventional insulin pumps (CGM + pump), or AID systems (CGM + AID).
Summary data might be made available on reasonable request via e-mail to the lead and corresponding authors.
The study was conducted between 10 February 2021 (date first participant signed informed consent) and 14 April 2021 (date last participant completed survey). Overall, 2,044 participants answered ≥1 type 1 diabetes–related question and reported CGM use status (Fig. 1).
Participant demographic and baseline clinical characteristics are shown in Table 1. Mean ± SD participant age was 43.0 ± 15.6 years for the total survey sample. The majority of participants were female (72.1% [n = 1,474]) and White (95.4% [n = 1,949]), with participants enrolled from the U.S. Mean ± SD time since diagnosis of type 1 diabetes was 26.3 ± 15.3 years. Of the total participants within the study, 1,875 (91.7%) were CGM users; 50.8% (953 of 1,875) of the CGM users used an AID system and 30.6% (574 of 1,875) used a conventional insulin pump. In the total survey sample, 2,029 (99.3%) participants had health insurance and 1,591 (77.8%) had private health insurance. Among all survey participants, the most recent mean ± SD self-reported HbA1c was 6.9% ± 1.1%.
Participant demographics and background characteristics
| Total survey sample, N | 2,044 |
| Age, years | 43.0 ± 15.6 |
| Age ≥65 years | 250 (12.2) |
| Female | 1,474 (72.1) |
| White | 1,949 (95.4) |
| Hispanic or Latino | 100 (4.9) |
| Have health insurance | 2,029 (99.3) |
| Have private health insurance | 1,591 (77.8) |
| BMI, kg/m 2 * | 27.7 ± 6.2 |
| Underweight (<18.5) | 22 (1.1) |
| Normal weight (18.5–24.9) | 794 (38.8) |
| Overweight (25–29.9) | 646 (31.6) |
| Obese (≥30) | 567 (27.7) |
| Duration since diagnosis, years | 26.3 ± 15.3 |
| Total daily dose of insulin, units/day | 49.7 ± 33.1 |
| Total daily dose of insulin/weight, units/kg/day | 0.6 ± 0.3 |
| Overall HbA1c† | 6.9 ± 1.1 |
| CGM users | 1,875 (91.7) |
| CGM + MDI | 339 (16.6) |
| CGM + pump | 574 (28.1)‡ |
| CGM + AID | 953 (46.6)‡ |
| Total survey sample, N | 2,044 |
| Age, years | 43.0 ± 15.6 |
| Age ≥65 years | 250 (12.2) |
| Female | 1,474 (72.1) |
| White | 1,949 (95.4) |
| Hispanic or Latino | 100 (4.9) |
| Have health insurance | 2,029 (99.3) |
| Have private health insurance | 1,591 (77.8) |
| BMI, kg/m 2 * | 27.7 ± 6.2 |
| Underweight (<18.5) | 22 (1.1) |
| Normal weight (18.5–24.9) | 794 (38.8) |
| Overweight (25–29.9) | 646 (31.6) |
| Obese (≥30) | 567 (27.7) |
| Duration since diagnosis, years | 26.3 ± 15.3 |
| Total daily dose of insulin, units/day | 49.7 ± 33.1 |
| Total daily dose of insulin/weight, units/kg/day | 0.6 ± 0.3 |
| Overall HbA1c† | 6.9 ± 1.1 |
| CGM users | 1,875 (91.7) |
| CGM + MDI | 339 (16.6) |
| CGM + pump | 574 (28.1)‡ |
| CGM + AID | 953 (46.6)‡ |
Data are means ± SD or n (%) unless otherwise indicated.
Height and weight were reported by 2,029 survey participants, with missing values for 15 participants.
HbA1c was self-reported by 1,975 participants within the past 12 months.
Nine CGM users included in total with pump type unknown.
Participant-reported mean HbA1c values were 6.8% (95% CI 6.8, 6.9) for CGM users and 7.5% (95% CI 7.3, 7.8) for non-CGM users, respectively. Overall, 59.6% (95% CI 57.3, 61.8) of participants using CGM and 35.5% (95% CI 28.4, 43.3) of those not using CGM met the glycemic target of HbA1c
Among all participants, ∼20% reported at least one SHE in the previous 12 months, with 7.8% (95% CI 6.1, 9.5) reporting one SHE and 12.0% (95% CI 10.4, 13.8) reporting two or more SHEs over that period (Fig. 2A). Overall, more than one SHE was reported by 10.8% (95% CI 9.1, 12.6) of CGM users and by 25.4% (95% CI 18.9, 33.1) of those who did not use CGM. The proportion of participants with at least one SHE was 16.6% for those using an AID system, 19.0% for those with conventional pumps, and 23.0% for those using MDI (Fig. 2B). SHE data were not available for 26 (1.3%) participants.
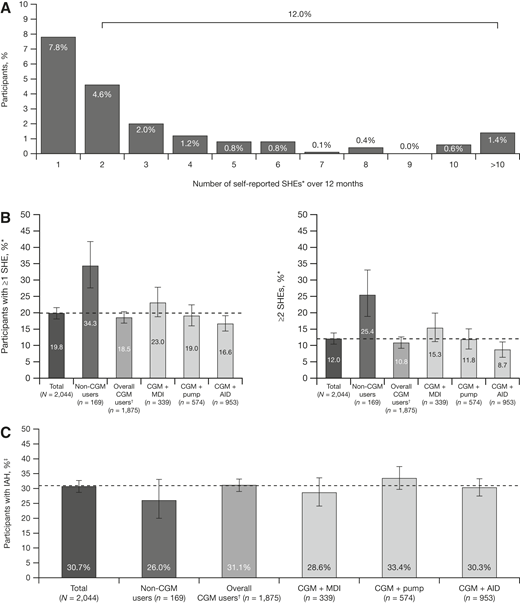
Proportion of participants in the total survey sample reporting SHEs in the past 12 months (A), one or more and two or more SHEs (B), and IAH (C). Error bars represent 95% CIs. The dotted line corresponds to the value of the total survey sample. *SHE was defined as a hypoglycemic event in the past 12 months where the patient experienced low blood glucose levels that they were unable to treat themselves and needed help from others. †Nine CGM users with pump type unknown. ‡Modified Gold score ≥4.

Proportion of participants in the total survey sample reporting SHEs in the past 12 months (A), one or more and two or more SHEs (B), and IAH (C). Error bars represent 95% CIs. The dotted line corresponds to the value of the total survey sample. *SHE was defined as a hypoglycemic event in the past 12 months where the patient experienced low blood glucose levels that they were unable to treat themselves and needed help from others. †Nine CGM users with pump type unknown. ‡Modified Gold score ≥4.
Based on modified Gold score, the proportion of all participants with IAH was 30.7% (95% CI 28.7, 32.7). The proportion of participants with IAH in the group who did not use CGM was 26% (95% CI 20.0, 33.1) compared with 31.1% (95% CI 29.0, 33.2) in the group who used CGM. Prevalence of IAH based on insulin delivery method was 30.3% (95% CI 27.5, 33.3) for participants using CGM + AID, 33.4% (95% CI 29.7, 37.4) for those using CGM + pump, and 28.6% (95% CI 24.1, 33.6) for those using CGM + MDI (Fig. 2C).
SHEs with IAH occurred in the survey sample despite the high penetration of advanced diabetes technologies (Fig. 3). The proportions of participants with at least one SHE and IAH were 7.8%, 9.9%, and 11.2% in the CGM + AID, CGM + pump, and CGM + MDI groups, respectively. In focusing on those with two or more SHEs and IAH, the proportions of participants were 4.7%, 6.6%, and 8.6% in the CGM + AID, CGM + pump, and CGM + MDI groups.
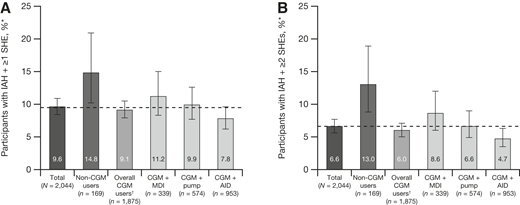
Proportion of participants in the total survey sample with one or more SHEs and IAH (A) or two or more SHEs and IAH (B). Error bars represent 95% CIs. The dotted line corresponds to the value of the total survey sample. *Nine CGM users with pump type unknown. †SHE was defined as a hypoglycemic event in the past 12 months where the patient experienced low blood glucose levels that they were unable to treat themselves and needed help from others.

Proportion of participants in the total survey sample with one or more SHEs and IAH (A) or two or more SHEs and IAH (B). Error bars represent 95% CIs. The dotted line corresponds to the value of the total survey sample. *Nine CGM users with pump type unknown. †SHE was defined as a hypoglycemic event in the past 12 months where the patient experienced low blood glucose levels that they were unable to treat themselves and needed help from others.
Recent guidelines recommend CGM as a first-line approach in the early management of type 1 diabetes (23). Furthermore, clinical trials and a growing number of real-world studies have shown the benefits of CGM use for glycemic control and rates of hypoglycemia among people with diabetes treated with insulin therapy (24). Results of a recent randomized trial by Laffel et al. (25) demonstrated a small but significant improvement in glycemic metrics when adolescent and young adult participants with type 1 diabetes used CGM compared with self-monitoring of blood glucose. Similarly, in a randomized trial where CGM was compared with self-monitoring of blood glucose in older adults, aged ≥60 years, the CGM group had a small, statistically significant reduction in hypoglycemia (26). Although these data show clear benefits through implementation of CGM in broad populations, a contingent of individuals with type 1 diabetes remain who continue to endure challenges in achieving clinical goals.
In the current study, glycemia, as assessed according to participant-reported HbA1c values from the past year, was used to help to evaluate the impact of CGM and insulin delivery technologies on the participants’ management of type 1 diabetes. Although more CGM than non-CGM users met the HbA1c target of 1c target. The proportion of participants who achieved HbA1c of 1c target of 1c target should be considered for those with a low risk of hypoglycemia. Our survey results show that despite being a highly engaged group who opted to respond to the survey, with the vast majority having private insurance, only a fraction (34.5%) achieved an HbA1c level
With respect to why IAH and SHEs persist despite the use of diabetes technology, this finding is likely multifactorial. Subcutaneously delivered insulin has a protracted pharmacodynamic profile inferior to endogenously secreted insulin (3). Namely, injected insulin cannot “turn off” as rapidly as it should, and glucose disposal still occurs even when glucose concentrations are dropping. Additionally, it is well documented that patients with type 1 diabetes lose their glucagon response to hypoglycemia early in the course of the disease (7). This creates a “perfect storm” for hypoglycemia in which patients are still prone to periods of excess insulin in the setting of glucagon deficiency and hypoglycemia occurs. While CGM and AID systems help mitigate these issues, they are not foolproof. The known CGM lag time in monitoring glucose levels can pose a challenge to a rapid algorithm correction response (28). Additionally, while AID algorithms generally help to minimize hyperglycemia and glucose variability, many individuals choose to override the systems to further optimize glycemic control, which often leads to insulin stacking, increased glucose variability, and hypoglycemia.
The prevalence of IAH identified in our study is consistent with ranges reported in published literature (29). Nearly one-third of study respondents (30.7%) reported IAH; this proportion was largely consistent across all analysis subgroups, independent of technology use. CGM devices are often recommended to individuals with IAH to aid their glucose monitoring and to potentially detect impending hypoglycemia (29). Interestingly, the proportion of respondents who had IAH was numerically lower among participants not using CGM (26.0%) compared with those using CGM (31.1%). The numerically larger proportion of IAH reported by CGM users in our study may reflect CGM being implemented in these participants for safety reasons due to prior SHE with IAH, although we can only speculate, since we did not collect information on timing of CGM initiation in relation to SHE occurrence. The evidence-based guidance for management of problematic hypoglycemia includes recommendations for education, technology use, and possible consideration of β-cell replacement when severe hypoglycemia continues to be concerning and potentially debilitating. Despite using AID, a proportion of respondents in our sample may still require targeted strategies to further mitigate hypoglycemia risk, given SHEs in the past year or classification as having IAH. Thus, even those using the most advanced diabetes technologies currently available could still be considered as potential candidates for β-cell replacement, where available.
Several limitations of this study should be considered. Participants who took part in this survey study were from the T1D Exchange online community, including the T1D Exchange Registry, a cohort of individuals with type 1 diabetes who tend to be highly engaged, have a high degree of technology use, and have historically been shown to be more likely to achieve glycemic targets (30). Survey respondents were predominantly female and White; however, results of sensitivity analyses with sex as a stratification factor were similar to those of the main analyses (data not shown). Furthermore, nearly 80% of participants had private insurance. As the outcomes of the survey were based on self-reports, the results may be subject to recall bias by participants. Additionally, information on timing of AID initiation and type of AID was not included in the survey. Therefore, whether SHEs occurred during AID treatment is unknown, and subgroup analyses based on AID type were not conducted. It should also be noted that individuals using CGMs and AIDs may potentially overtreat and/or overreport SHEs due to sensor alarms. In future studies investigators should both aim to provide descriptive analyses and include statistical hypothesis testing and aim for more diversity among participants populations within a wider age-group to account for both pediatric and older individuals with type 1 diabetes. The sample included may not fully represent the general population, globally, as many individuals with type 1 diabetes do not have access to a specialist or to the advanced technologies described within this study (31). As such, the conclusions drawn from this sample, a self-selected group of participants, may not be directly generalizable to the overall population with type 1 diabetes, and outcomes are likely to be even less favorable with respect to glycemia and rates of severe hypoglycemia.
Despite high rates of diabetes technology adoption among study participants (92% using CGM and 47% using CGM + AID), more than one-third of survey respondents reported not being able to reach glycemic targets, with up to 20% having at least one SHE in the past 12 months and 30% having IAH. Given these results, educational initiatives continue to be important for all individuals with type 1 diabetes, and the development of novel therapeutic options and strategies, including bihormonal AID systems and β-cell replacement, will be required to enable more of these individuals to meet treatment goals.
Acknowledgments. Editorial support and coordination were provided by Nathan Blow of Vertex Pharmaceuticals, who may own stock or stock options in the company. Medical writing support and editing support were provided under the guidance of the authors by Terrance Ku and Lauren Smith, of Complete HealthVizion, IPG Health Medical Communications, funded by Vertex Pharmaceuticals.
J.P. is an editor of Diabetes Care but was not involved in any of the decisions regarding review of the manuscript or its acceptance.
Funding and Duality of Interest. This study was funded by Vertex Pharmaceuticals. J.L.S. has received grants or contracts from JDRF, National Institute of Diabetes and Digestive and Kidney Diseases, Jaeb Center for Health Research, Insulet, Medtronic, Provention Bio, and Abbott paid to her institution; has received consulting fees from Abbott; has received payment or honoraria from Insulet, Medtronic Diabetes, and Zealand Pharma; has participated in advisory boards for Bigfoot Biomedical, Cecelia Health, Insulet, Medtronic Diabetes, Novo Nordisk, and Vertex Pharmaceuticals; and reports owning stock/stock options of StartUp Health T1D Moonshot. L.M.L. has received consulting fees from Vertex Pharmaceuticals. J.L., W.W., J.B., K.S.C., and D.F. were employees of T1D Exchange during the course of the study. L.T. is a board member for COJECO, was an employee at Vertex Pharmaceuticals during the course of the study, and is currently an employee at GlaxoSmithKline. J.G. has received grants or contracts from Avotres, Dompé Farmaceutici, and Imcyse paid to his institution; has received consulting fees from Avotres, Imcyse, Dompé Farmaceutici, Regeneron, Vertex Pharmaceuticals, Diamyd Medical, and Current Health; has received payment or honoraria from Foundation for Advanced Education in the Sciences at the National Institutes of Health; and reports owning stock/stock options of Vertex Pharmaceuticals. T.L., K.H., and K.C. are employees at Vertex Pharmaceuticals and own stocks/stock options of Vertex Pharmaceuticals. R.B. has received consulting fees from Abbott Diabetes Care, Ascensia Diabetes Care, Bigfoot Biomedical, DexCom, MannKind, Medtronic, Novo Nordisk, Sanofi, and United Healthcare paid to HealthPartners Institute; has received payment or honoraria from Sanofi and Vertex Pharmaceuticals paid to HealthPartners Institute; has received support to attend meetings from Abbott Diabetes Care, Ascensia Diabetes Care, CeQur, Eli Lilly, Embecta, MannKind, Novo Nordisk, Roche GmbH, Sanofi, Vertex Pharmaceuticals, and Zealand Pharma; and has participated in advisory boards for Abbott Diabetes Care, CeQur, Eli Lilly, embecta, Hygieia, Roche GmbH, and Zealand Pharma. J.P. has received consulting fees from Novo Nordisk, Sanofi, Eli Lilly, Carmot Therapeutics, Diasome, Kriya, and Biomea Fusion. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors contributed to the development of the study design. J.L., W.W., J.B., K.S.C., and D.F. acquired study data. J.L., W.W., J.B., K.S.C., D.F., T.L., L.T., and K.H. conducted the analyses of the study data. All authors were involved in interpreting the data, reviewing and drafting the manuscript, and approving the final version of the manuscript to be published. All authors agreed to be accountable for the work in the manuscript. K.H. and J.P. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 82nd Scientific Sessions of the American Diabetes Association, New Orleans, LA, 3–7 June 2022, and at the 58th Annual Meeting of the European Association for the Study of Diabetes, 19–23 September 2022, Stockholm, Sweden.
Handling Editors. The journal editor responsible for overseeing the review of the manuscript was Mark A. Atkinson.
See accompanying article, p. 918.